Polyethylene 2,5-furandicarboxylate (PEF) offers sustainable alternative to replace polyethylene terephthalate (PET). 2,5-Furandicarboxilic acid (FDCA) is one of the monomers needed to produce PEF. It is a dicarboxylic acid on a five membered aromatic furan ring, similar to the six membered phenyl group of terephthalic acid (PTA), one of the monomer of PET.
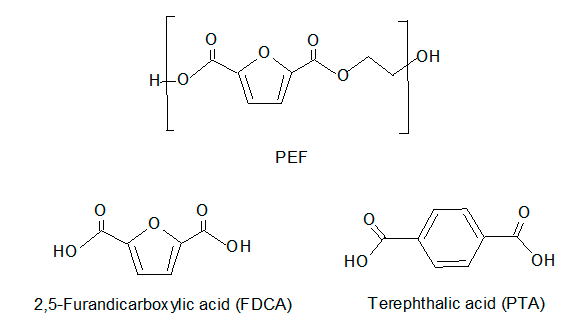
PTA is made from p-xilene and ethylene glycol obtained from oil refining and from oil cracking, respectively. However, FDCA is obtained from oxidation of 5-furfural that is synthesized via fructose dehydration. Therefore, PEF could be obtained from renewable feedstock since ethylene glycol can also be formed from bioethanol. FDCA is also found in human urine (3-5 mg/day.person). FDCA diethyl ester has anesthetic action and other of its derivatives have antibacterial properties.
PEF is expected to have better performance than PET as it has a higher glass transition and lower melting point. PEF may also be a superior material for bottles and films for food packaging due to its improved gas barrier. FDCA/PEF commercial scale and demo plants are operating or under planning. However, several barriers1 are preventing the full potential of FDCA from being unbarred. For instance feedstock cost, resources for production as catalyst, solvent and energy. FDCA purity is also an impediment.
1.- V.Haas ,J. Wenger , L. Ranacher , N. Guigo, A. F. Sousa ,T. Stern, Sustainable Production and Consumption 31 (2022) 370–383





