Electroluminescence is the opposite process of voltaic transformation (solar cell: light to electrical energy conversion); namely, electrical energy is converted into light. The corresponding device is a light-emitting diode, which in the case one or more of its constituents layers are organic compounds, are named organic light-emitting diode (OLED). OLEDs are currently used in small-screen applications such as mobile phones, digital cameras, and PDAs. The organic constituents may be conductive polymers as Poy(N-vinylcarbazole) (PVK).
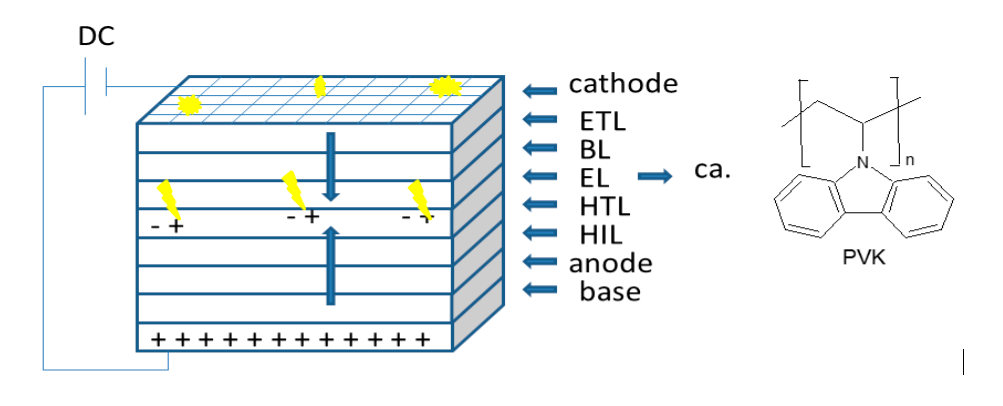
OLEDs are made1 of several layers where the top and bottom ones are connected to a DC power (2-10 V). Layers are divided into pixels where light can be on or off. Once the DC power is on, electrons (-) at the cathode move down throw the electron transport and blocking layers (ETL & BL) and holes (+) move up from the anode through the hole injection and the hole transport layers (HIL & HTL). Electrons and holes encounter liberating energy as light2 at the Emissive layer (EL). External applied voltage controls which pixels light up and which stay dark. A thin-film transistors (TFTs) circuit (not shown in figure) may also be used to switch on pixels to form the corresponding image. The BL and HIL control electron and hole flow, respectively.
1.- M. L. P. Reddy, K. S. Bejoymohandas, J. Photochem. Photobiol. C Photochem. Rev.,29 (2016) 29-47 doi: 10.1016/j.jphotochemrev.2016.10.001.
2.- N. Sain, D. Sharma, and P. Choudhary, Int. J. Eng. Appl. Sci. Technol., 4, 11 (2020) 587-591 doi: 10.33564/IJEAST.2020.v04i11.103.





