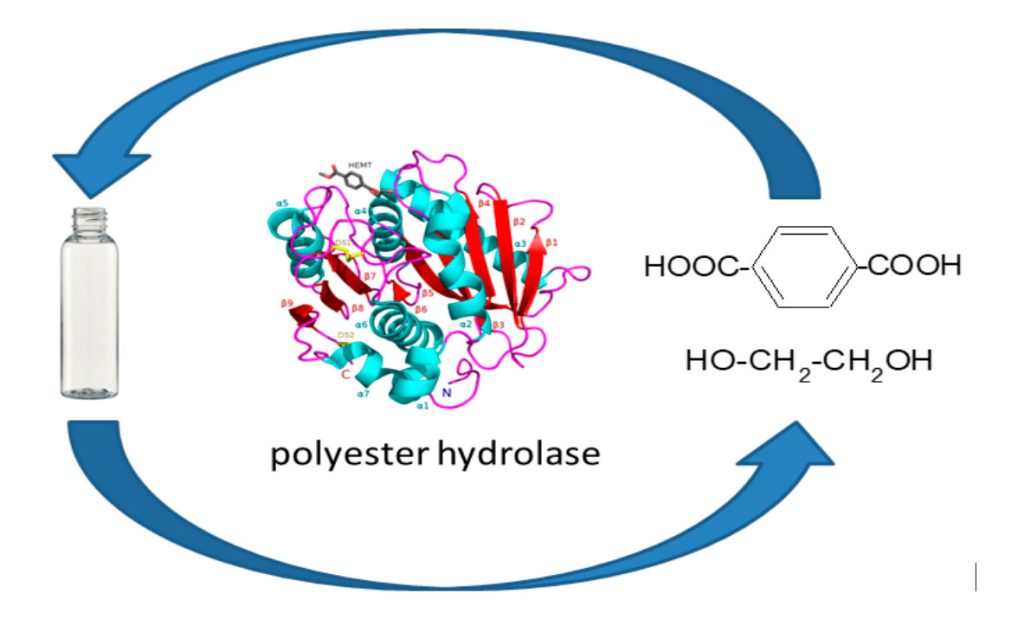
German researcher from Leipzig University claim1 to have found an enzyme that is able to degrade crystalline PET (polyethylene terephthalate). Accordingly, a polyester hydrolase named PHL7 (isolated from a compost metagenone) degrades amorphous and crystalline PET, releasing terephthalic acid and ethylene glycol that have been used to successfully synthesize virgin PET, completing in this way the sustainable PET recycling (Figure). PHL7 hydrolyzes amorphous PET films, releasing 91 mg of terephthalic acid per hour and 6.8 μm h−1 of PET film. Also, in less than 24 h, 0.6 mgenzyme gPET−1 completely degrades post-consumer thermoform PET packaging in an aqueous buffer at 70 °C.
The Ser-Asp-His triad and an alpha/beta-hydrolase fold are identified as the active site and enzyme structure, respectively of PHL7 and LCC (a previously tested2 active enzyme) in the PET ester hydrolysis. Presence of Amino acid Leucine at position 210 of PHL7 is also determining factor in the enzyme catalytic activity.
 1.- C. Sonnendecker, J. Oeser,P. K. Richter,P. Hille,Z. Zhao,C. Fischer,H.Lippold,P.Blázquez-Sánchez,F. Engelberger,C. A. Ramírez-Sarmiento,T. Oeser,Y. Lihanova, R. Frank, H-G. Jahnke, S. Billig, B. Abel, N. Sträter, J. Matysik, W. Zimmermann ChemSusChem (2022) ,15, doi.org/10.1002/cssc.202101062
1.- C. Sonnendecker, J. Oeser,P. K. Richter,P. Hille,Z. Zhao,C. Fischer,H.Lippold,P.Blázquez-Sánchez,F. Engelberger,C. A. Ramírez-Sarmiento,T. Oeser,Y. Lihanova, R. Frank, H-G. Jahnke, S. Billig, B. Abel, N. Sträter, J. Matysik, W. Zimmermann ChemSusChem (2022) ,15, doi.org/10.1002/cssc.202101062
2.- V. Tournier, C. M. Topham, A. Gilles, B. David, C. Folgoas, E. Moya-Leclair, E. Kamionka, M.-L.Desrousseaux, H. Texier, S. Gavalda, M. Cot, E.Guémard, M. Dalibey, J. Nomme, G. Cioci, S. Barbe, M. Chateau, I. André, S. Duquesne, A. Marty, Nature (2020), 580,216–21